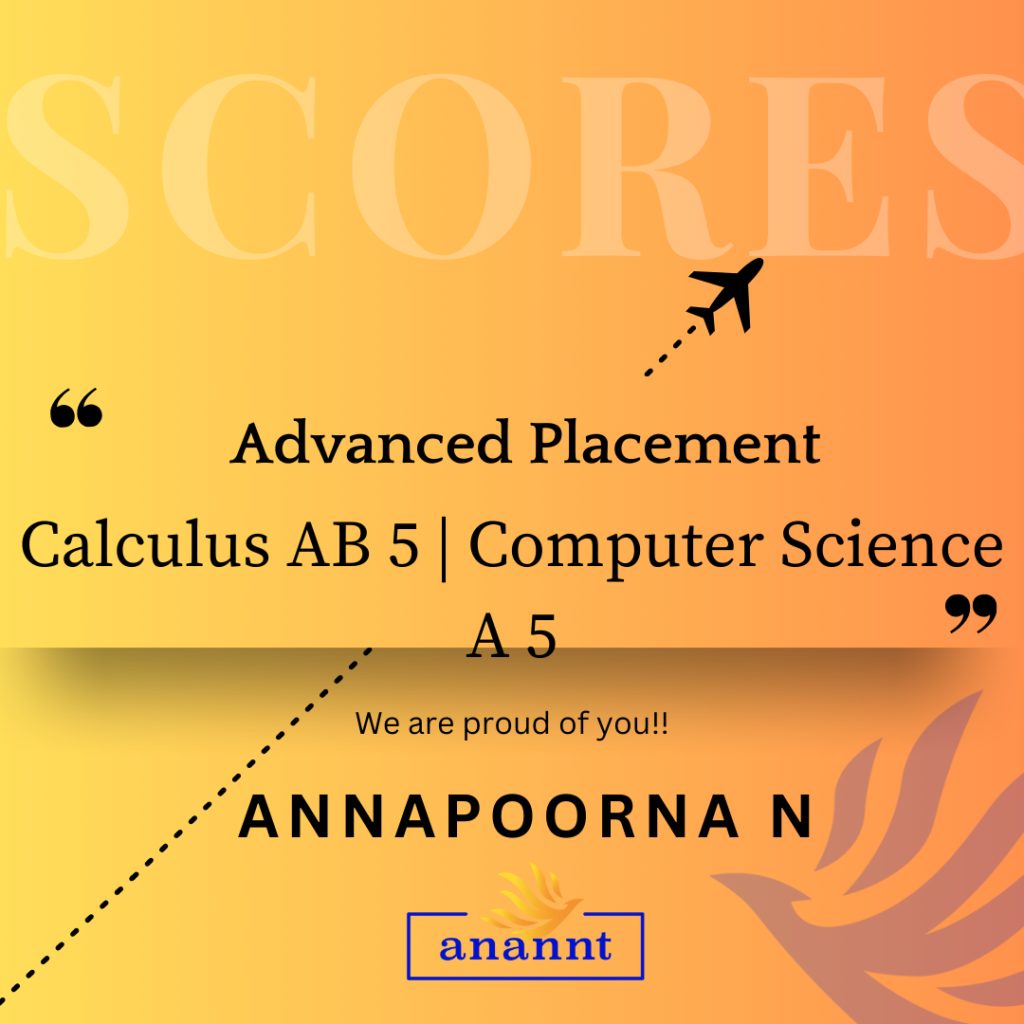
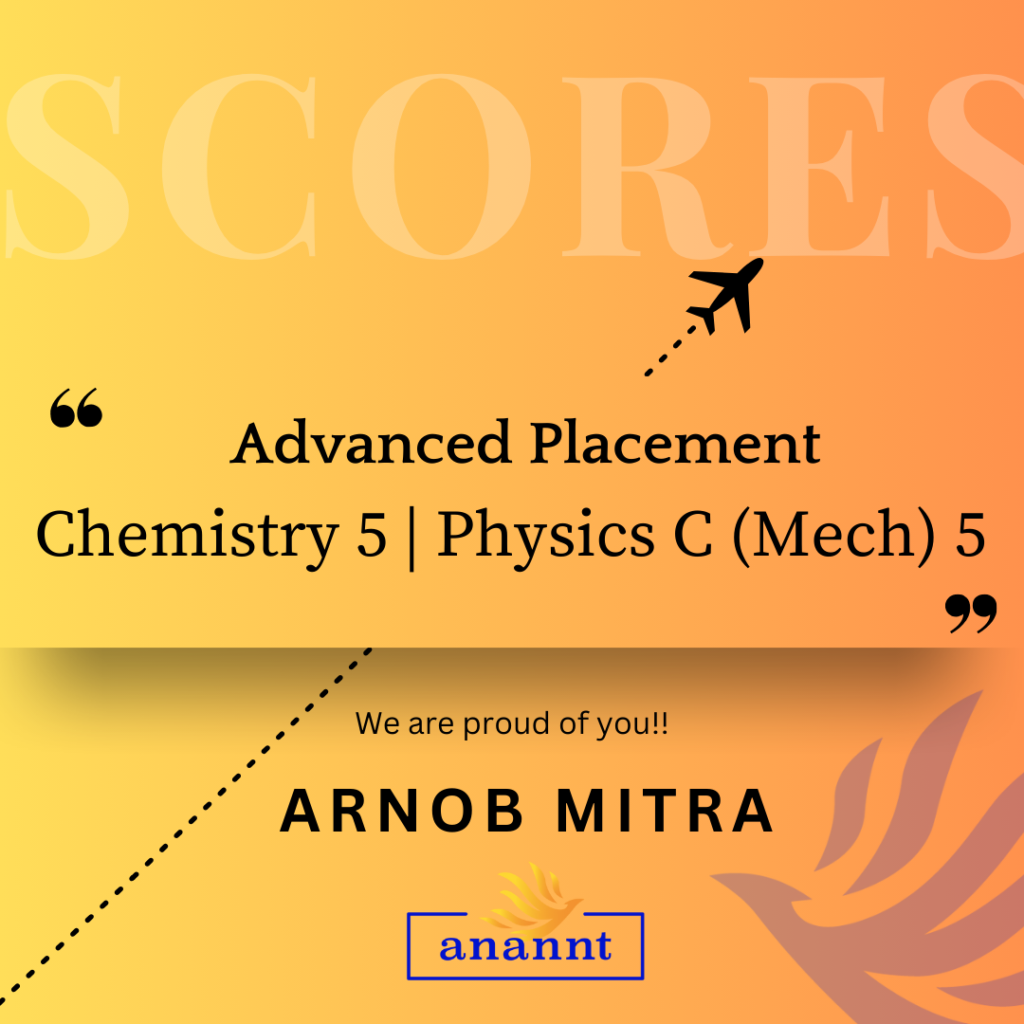
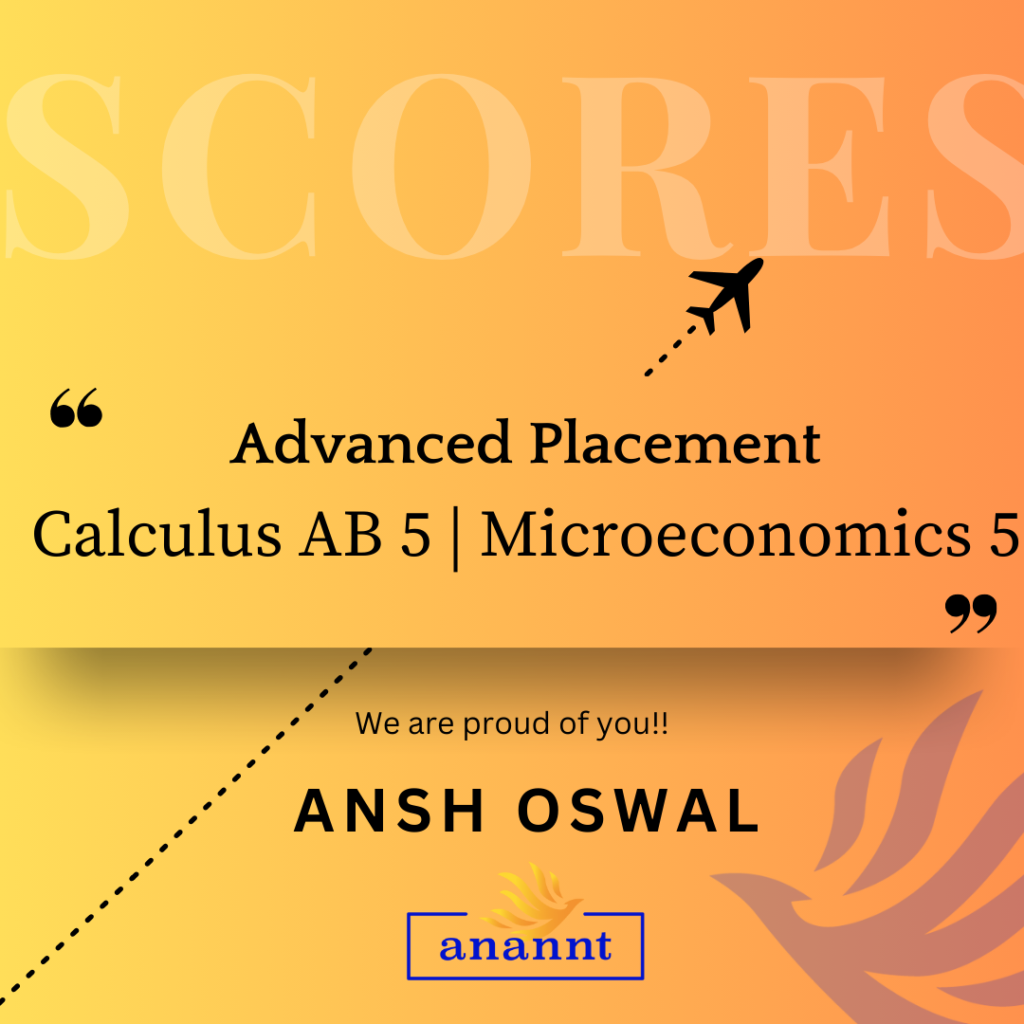
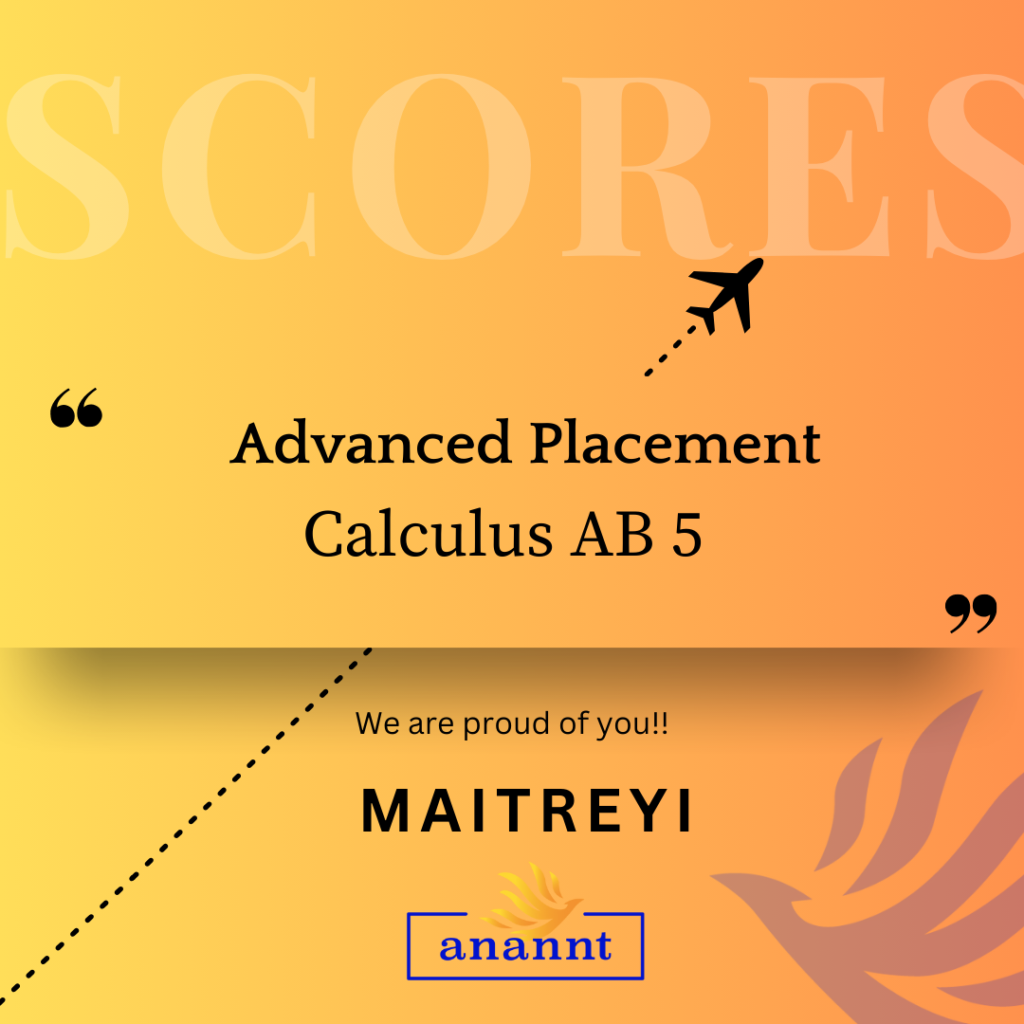
AP Chemistry: Unlock Your Dream University Pathway With Anannt Education
Success Starts Here
Don’t Miss Out! Chat with our advisior on WhatsApp (+971) 585709551 to learn more about AP Chemistry.
Enroll Now For Free Demo Class
Meet Our Faculty
Meet Prerna Sharma, our esteemed Head of Verbal at Anannt Education and a seasoned trainer in GRE verbal, GMAT, AP, and SAT with an impressive 18+ years of experience. Prerna holds advanced degrees in English Literature, specializing in American Literature, which she seamlessly integrates into her diverse teaching portfolio, including AP Biology, Environmental Sciences, Psychology, chemistry,AP US History and Microeconomics. Her teaching methodology is both innovative and engaging, blending her literary expertise with a deep understanding of each subject to foster critical thinking and a love for learning. Prerna’s approach not only elevates students’ academic performance but also prepares them for the interconnected world of knowledge. Join her classes for an enriching and transformative educational experience.
Elevate AP Journey: With Anannt Education
Course Structure 📚
- 40+ Hours of Learning 🕒: Interactive sessions, in-person & online.
- Small Batches 👥: Max 10 students for personalized focus.
- Custom Plans 🎯: Diagnostic tests tailor your study journey.
- Regular Assessments ✔️: Advanced testing portal for constant improvement.
Study Material 📖
- Expert Tutors 👩🏫: Personalized guidance from seasoned pros.
- Rich Resources 📈: Detailed notes, video tutorials, and topic-wise tests.
- Mock Tests 📝: 5 full-length exams for a real test experience.
- Online Portal 💻: Quizzes and explanations for deeper learning.
Anannt Advantage ✨
- Dedicated Mentors 🌟: Celebrated for exceptional mentorship.
- Holistic Approach 🔄: Blends guidance, support, and materials for success.
- Proven Methods 📊: Achieve excellence with our tested teaching strategies.
- Unlock Potential 🔓: Realize your ambitions and excel in AP Chemistry
AP Chemistry Syllabus
Unit 1: Atomic Structure and Properties (7%–9%)
- 📏 Moles and molar mass: Understand the concept and calculations.
- 🔍 Mass spectroscopy: Determine atomic mass via mass spectrometry.
- 🧪 Elemental composition: Calculate percent composition from molecular formulas.
- 🔬 Mixtures: Identify composition and mixture types.
- ⚛️ Atomic structure: Explore protons, neutrons, electrons, and electron configurations.
- 🌈 Photoelectron spectroscopy: Use it to determine electron configurations.
- 📈 Periodic trends: Discover properties changes across the periodic table.
- 🔌 Valence electrons: Learn their role in forming ionic compounds.
Unit 2: Molecular and Ionic Compound Structure and Properties (7%–9%)
- 🌐 Chemical bonds: Ionic, covalent, and metallic bonds.
- 💪 Intramolecular forces: Understand forces within molecules.
- 🔳 Ionic solids: Study their crystal lattice structure.
- 🛠 Metals and alloys: Dive into their structure and formation.
- 🎨 Lewis diagrams: Use them for representing molecules.
- ↔️ Resonance and formal charge: For predicting electron arrangements.
- 📐 VSEPR and hybridization: Predict molecular geometry.
Unit 3: Intermolecular Forces and Properties (18%–22%)
- 🤲 Intermolecular forces: London dispersion, dipole-dipole, hydrogen bonding.
- 🌡 States of matter: Relation to intermolecular forces.
- 🚗 Kinetic molecular theory: Describes motion in solids, liquids, gases.
- 💧 Solutions: Understand types and solubility.
- 🌞 Photoelectric effect: Electron emission from metal surfaces.
Unit 4: Chemical Reactions (7%–9%)
- 🔄 Introduction to reactions: Understanding bond formation and breaking.
- 🧮 Net ionic equations: Focus on participants in reactions.
- 📝 Reaction representations: Word equations, formulas, and balancing.
- 🔄 Physical vs. chemical changes: Recognize the signs.
- ⚖️ Stoichiometry: Relationships between reactants and products.
- 🎭 Reaction types: Synthesis, decomposition, exchange, redox.
Unit 5: Kinetics (7%–9%)
- ⏱ Reaction rate: Measure and calculate reaction speeds.
- 📊 Rate laws: Describe the effect of concentration on reaction rate.
- 🧩 Elementary reactions: Simplest steps in mechanisms.
- 💥 Collision model: How reactions occur through molecule collisions.
- 🛤 Reaction mechanisms: Series of steps in reactions.
- ⚡ Energy profiles: Identify and interpret multistep reaction energy.
Unit 6: Thermodynamics (7%–9%)
- 🌞 Endothermic vs. exothermic: Recognize and calculate heat in reactions.
- 🌡 Heat transfer: Understand thermal equilibrium.
- 🔥 Calorimetry: Measure reaction heat.
- 🍦 Phase changes: Calculate energy involved in phase transitions.
- 📊 Enthalpy of reaction and formation: Calculate reaction heat.
- 🔄 Hess’s law: Enthalpy change consistency in reactions.
Unit 7: Equilibrium (7%–9%)
- ⚖️ Basics of chemical equilibrium: Understanding forward and reverse reaction rates.
- 🧮 Equilibrium constant (Kc): Calculate and predict reaction directions.
- 🌊 Solubility equilibria: Calculate solubility product constant (Ksp).
- 🔄 Le Châtelier’s principle: System adjustments to maintain equilibrium.
Unit 8: Acids and Bases (11%–15%)
- 🍋 Basics of acids and bases: Definitions and reactions.
- 🧪 pH and pOH: Calculate for strong acids and bases.
- 🛡 Acid-base reactions and buffers: Equation balancing and pH maintenance.
- 🔬 Molecular structure: Impact on acidity and basicity.
- 📈 pH and pKa: Understand their relationship.
Unit 9: Applications of Thermodynamics (7%–9%)
- ❄️ Entropy: Concept and relation to system disorder.
- 🔋 Gibbs Free Energy: Determine reaction favorability.
- 🎲 Thermodynamic vs. kinetic control: Impact on reaction outcomes.
- 🔌 Electrochemistry: Galvanic cells, electrolysis, and Faraday’s law.
🎓 AP Chemistry Exam Structure 🎓
Section I: Multiple Choice (50% of Total Score)
- Duration: 1hr 30mins.
- Questions: 60, a mix of individual and sets related to diagrams or data presentations.
- Skills Tested:
- Analysis and interpretation of chemical properties/phenomena. 🕵️♂️
- Design of experiments to test theories. 🧫
- Problem-solving using mathematical relationships. 🧮
- Making/justifying scientific claims with evidence. 📚
Section II: Free Response (50% of Total Score)
- Duration: 1hr 45mins.
- Questions: Three long essays (10 points each) + four short answers (4 points each).
- Skills Tested:
- Deep analysis of chemical models/phenomena. 📊
- Experiment design and data representation. 📈
- Mathematical problem-solving. 🔢
- Scientific claim justification with evidence. 🏆